The Hydroxychloroquine Controversy: Examining Efficacy and Risks
Written on
Chapter 1: Understanding Hydroxychloroquine
Hydroxychloroquine has emerged as a hot topic in discussions about COVID-19 treatment. As previously noted, identifying an effective remedy could greatly change how we handle this pandemic. During his briefing on April 5, President Trump questioned the public, “What do you have to lose?” while advocating for wider access to this medication through legislation designed for patient rights.
Laboratory studies indicate that hydroxychloroquine can inhibit the entry of coronaviruses, such as SARS and COVID-19, into human cells. However, results from test tubes do not always translate to real-world effectiveness. For instance, hydroxychloroquine did not reduce viral loads in mice during SARS studies. Additionally, individuals who regularly take hydroxychloroquine for conditions like lupus or arthritis have still contracted COVID-19, indicating that it is not a preventive measure. Nevertheless, hydroxychloroquine remains a potential therapeutic option and warrants further investigation.
I remain optimistic that hydroxychloroquine, particularly when used alongside azithromycin or zinc, may demonstrate efficacy in the ongoing clinical trials. The desire for a successful treatment is universal, but caution is necessary. Hydroxychloroquine, especially in combination with azithromycin, has been linked to serious side effects, including visual disturbances and cardiac issues like arrhythmias. As per the Hippocratic Oath, “do no harm,” physicians are understandably reluctant to prescribe medications known to cause adverse effects without solid evidence from rigorous studies. Recently, hospitals in France and Sweden have suspended their clinical trials involving hydroxychloroquine due to heightened cardiac risk without demonstrated benefits.
For up-to-date information on hydroxychloroquine, I recommend following Derek Lowe, PhD, a pharmacologist who maintains a blog that critically reviews emerging data. The current body of evidence is, in short, inconclusive. Many studies are still in the “pre-print” phase, meaning they have not yet undergone peer review, which is a crucial step before formal publication. One recent pre-publication abstract by Dr. Didier Raoult caught my attention and was also highlighted by major media outlets like The New York Times and Fox News.
In brief, Dr. Raoult is a polarizing figure advocating for the use of hydroxychloroquine combined with azithromycin (HCQ-AZ) to treat COVID-19. He published promising results on March 20, reporting positive outcomes in a cohort of 42 patients, 26 of whom received HCQ-AZ. This study was met with immediate scrutiny due to its methodology; Dr. Raoult was involved in the selection of patients for HCQ-AZ versus placebo, and his analysis excluded any patients with poor outcomes. A review of the data later indicated no advantages when considering these methodological flaws. The International Society of Antimicrobial Chemotherapy commented that the study did not adhere to their expected standards, particularly regarding patient inclusion criteria and safety protocols.
The initial findings piqued President Trump’s interest, prompting him to tweet about hydroxychloroquine on March 21 and reference it multiple times during his briefings.
The Study
On April 10, Dr. Raoult shared a pre-publication abstract and data table summarizing his findings from a study involving over 1,000 patients. Below are the key findings (highlighted) alongside the methodological questions that still require clarification:
1,061 out of 3,165 patients diagnosed with COVID-19 through PCR testing met the study’s inclusion criteria. What were these criteria? Why were the other 2,000 patients excluded? Notably, this study lacked a control group. A robust clinical trial should be randomized and double-blinded, ensuring neither the patient nor the investigator knows which treatment group they belong to. Without a control group, it is impossible to ascertain whether the observed outcomes stem from the treatment or simply reflect the characteristics of the selected patient population.
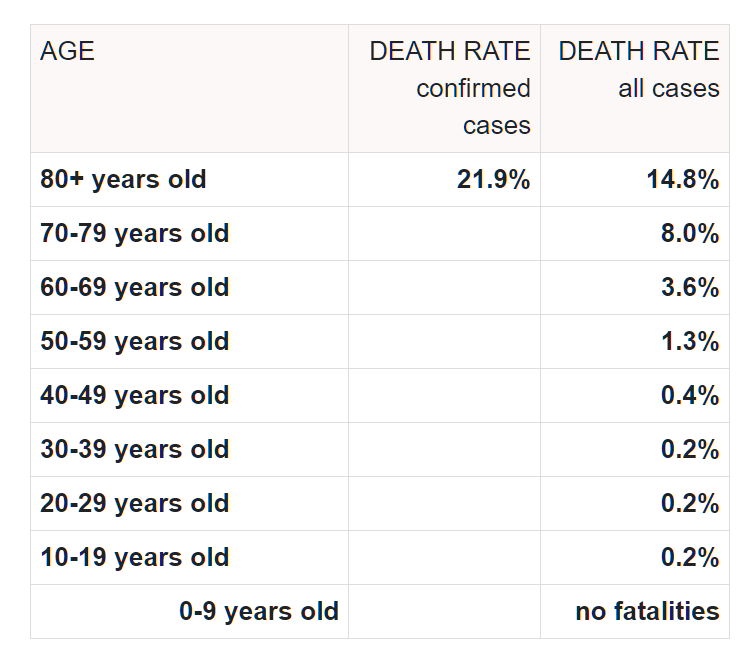
The mean age of participants was 43.6 years with a standard deviation of 15.6. Dr. Raoult later claimed that HCQ-AZ is a safe and effective treatment for older patients. However, the standard deviation suggests that 84% of participants were under the age of 59.2. Current global data indicates that the expected mortality rate for individuals under 60 ranges from 0.2% to 1.3% for those aged 50–59. This implies that the study cohort was younger and healthier than average, which could skew the outcomes favorably regardless of treatment.
A “good clinical outcome and virological cure was achieved in 973 patients within 10 days (91.7%).” This outcome was defined as the clearance of viral shedding by day 10 and avoidance of hospitalization beyond 10 days, transfer to ICU, or death. Notably, 4.4% of patients exhibited prolonged viral shedding past day 10, although all but one cleared the virus by day 15.
Poor outcomes were reported in 46 patients (4.3%), defined as hospitalization beyond 10 days (31 patients), ICU transfer (10 patients), or death (5 patients). The deceased patients ranged from ages 74 to 95, and of the 31 hospitalized for more than 10 days, 16 remained hospitalized at the time of publication. The overall mortality rate was 0.5%, with an additional 1.6% of patients still hospitalized.
Worse outcomes were associated with older age, initial severity of illness, and low HCQ serum levels. Additionally, patients taking beta-blockers and angiotensin II receptor blockers experienced poorer outcomes. These observations align with global clinical experiences.
Mortality among patients receiving more than three days of HCQ-AZ therapy was reported to be lower than among patients on other treatment regimens at the same hospital (IHU) and within the Marseille public hospitals. However, this claim lacks validation from comparative data across treatment regimens and hospitals.
The data table indicated that complete data was only available for 928 patients, meaning the initially reported figure of 1,061 is misleading, as 56 patients were excluded for being asymptomatic and 77 had incomplete data.
Conclusion
Dr. Raoult’s latest publication appears promising at first glance, claiming a 91.7% cure rate within 10 days and only 0.5% mortality. However, the absence of a control group and the lack of clarity regarding patient selection raise significant concerns. If the study cohort was unintentionally or intentionally composed of younger, healthier individuals, the impressive outcomes may not be attributable to hydroxychloroquine. We require access to the complete manuscript to determine whether this abstract is genuinely hopeful or potentially misleading.
The gold standard for clinical evaluation remains a double-blind, randomized control trial, and I sincerely hope we receive data from such trials in the near future.
Note from the Editors: Towards Data Science is a Medium publication focused on data science and machine learning. We are not health professionals or epidemiologists, and the views expressed in this article should not be construed as professional medical advice. For further information on the coronavirus pandemic, you can click here.
Chapter 2: The Great Hydroxychloroquine Debate
This video, titled "Who Won The Great Debate on Hydroxychloroquine at ACR 2018," explores the discourse surrounding hydroxychloroquine, delving into the arguments presented during the debate.
The second video, "The Debate Over the Effectiveness of the Drug Hydroxychloroquine," further examines the discussions regarding the efficacy and safety of hydroxychloroquine as a treatment option.