Understanding Stroke Risk Related to mRNA Vaccines: Latest Insights
Written on
Chapter 1: Background on Vaccine Safety
Recent research has called into question earlier claims regarding the connection between mRNA vaccines and stroke risk. Earlier this year, both the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) released preliminary findings indicating a possible rise in stroke risk associated with the simultaneous administration of COVID-19 (bivalent mRNA) and influenza (high-dose or adjuvanted) vaccines.
A detailed examination of this topic can be found in my previous article: “Stroke and mRNA Vaccine: An In-Depth Analysis.” It was noted that individuals over 65 years of age exhibited a 1.47-fold increase in the risk of ischemic stroke following Pfizer’s bivalent mRNA vaccination when evaluated through the Vaccine Safety Datalink (VSD) database.
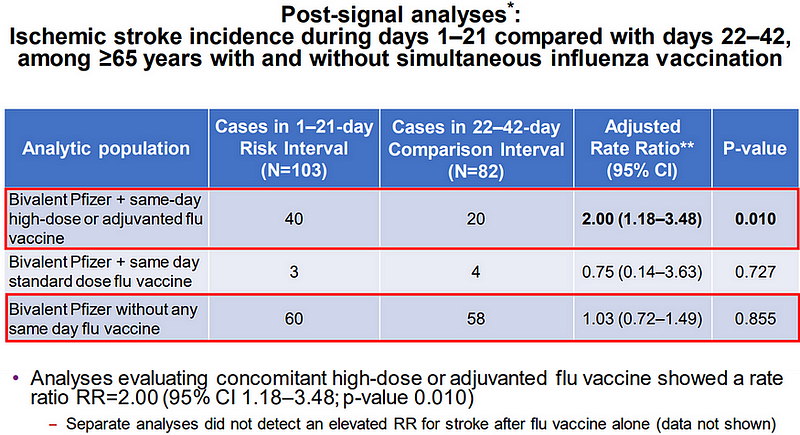
Further scrutiny indicated that this risk was particularly pronounced among those who received both vaccines on the same day, with the risk of stroke doubling (Figure 1). Notably, no increased risk was identified for those who received only the bivalent mRNA vaccine, the standard-dose influenza vaccine, or the influenza vaccine alone. This suggests that heightened immune responses triggered by strong vaccination stimuli may lead to strokes in vulnerable populations.
However, given that this was the first analysis to report such a risk, it was premature to draw definitive conclusions, leaving open the possibility of a false alarm.
Figure 1. The top red box illustrates a two-fold increased stroke risk during the 1-21 day period following the combination of Pfizer’s bivalent mRNA and high-dose influenza vaccination compared to the 22-42 day period. Source: FDA Vaccines and Related Biological Products Advisory Committee.
Chapter 2: New Findings from Recent Studies
After several months of silence, new research has emerged to provide clarity on this issue. The study titled “BA.1 Bivalent COVID-19 Vaccine Use and Stroke in England,” conducted by Andrews et al. from the U.K. Health Security Agency and the London School of Hygiene and Tropical Medicine, was published in the Journal of American Medical Association (JAMA) Network on June 15, 2023.
This research employed a similar self-controlled case series methodology as the CDC's analysis, comparing the stroke risk during the 1–21 day period post-vaccination (risk period) with a control period spanning 22–42 days.
In this study, 14.6 million individuals aged over 50 who received the bivalent mRNA vaccine in the U.K. National Immunisation Management System were analyzed. Of these, 983 individuals (approximately 11.7%) received an adjuvanted influenza vaccine on the same day, predominantly those aged over 65. By the study's conclusion, there were 6,882 cases of ischemic stroke and 1,510 cases of hemorrhagic stroke recorded.
The results indicated that for participants over 50 who received the bivalent mRNA vaccine (Pfizer or Moderna), either alone or alongside the adjuvanted influenza vaccine, no significant changes in the risk of ischemic or hemorrhagic stroke were observed in the risk compared to the control period. This finding was consistent among those older than 65 years as well.
Chapter 3: Interpreting the Data
While the findings from the U.K. study are reassuring, it is important to consider the limitations of the research. Firstly, the bivalent mRNA vaccine examined targeted the BA.1 Omicron variant, which differs from the BA.5 variant focused on in the U.S. CDC study. Nonetheless, there is no evidence to suggest that the safety profiles of the two vaccine types would differ significantly.
Secondly, only a minor fraction (~10%) of participants in the U.K. study received both vaccines on the same day, a limitation shared by the U.S. CDC analysis. Thirdly, both studies operated under the premise that the risk of stroke is acute, likely manifesting within 21 days post-vaccination, which is reasonable given the typical duration of immune responses to vaccines.
Overall, while the U.K. and U.S. CDC studies share similar designs, they produced conflicting results. Therefore, the U.S. CDC's observation of an increased stroke risk following COVID-19 and influenza co-vaccination may indeed have been a false alarm. If such co-vaccination truly elevates stroke risk, it should manifest consistently across different populations.
Moreover, Health Canada and the Public Health Agency of Canada (PHAC) reported no elevated risks associated with vascular events, including stroke, as of January 2023, after distributing over 7 million bivalent mRNA vaccines. Although this data does not account for influenza co-vaccination, Canada administered flu shots concurrently with the bivalent mRNA vaccines.
It is plausible that confounding factors may have influenced the results of the U.S. CDC study, such as variations in health behaviors where individuals with poorer health are more likely to seek out the bivalent mRNA vaccine. The statistical significance of these findings could also be coincidental.
If you’ve read this far, I appreciate your interest. To stay updated, consider subscribing to my Medium email list. If you're interested in becoming a member for unlimited access to Medium, using my referral link will provide me with a small commission at no additional cost to you. Any tips are also greatly appreciated as they support my work.
Video Description: A detailed discussion about the 'Kraken' variant and its association with vaccine-related stroke risks, featuring Sandra Fryhofer, MD.
Video Description: An overview of the CDC's vaccine safety data link and the flagged possible brain stroke risks associated with recent vaccinations.